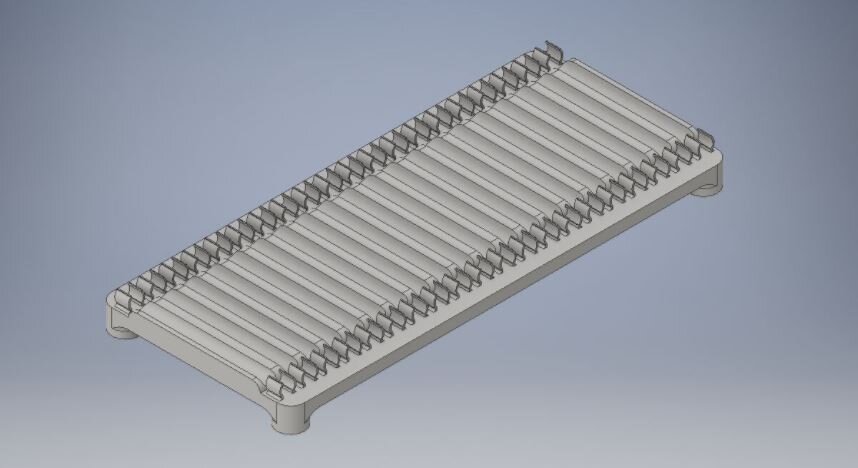
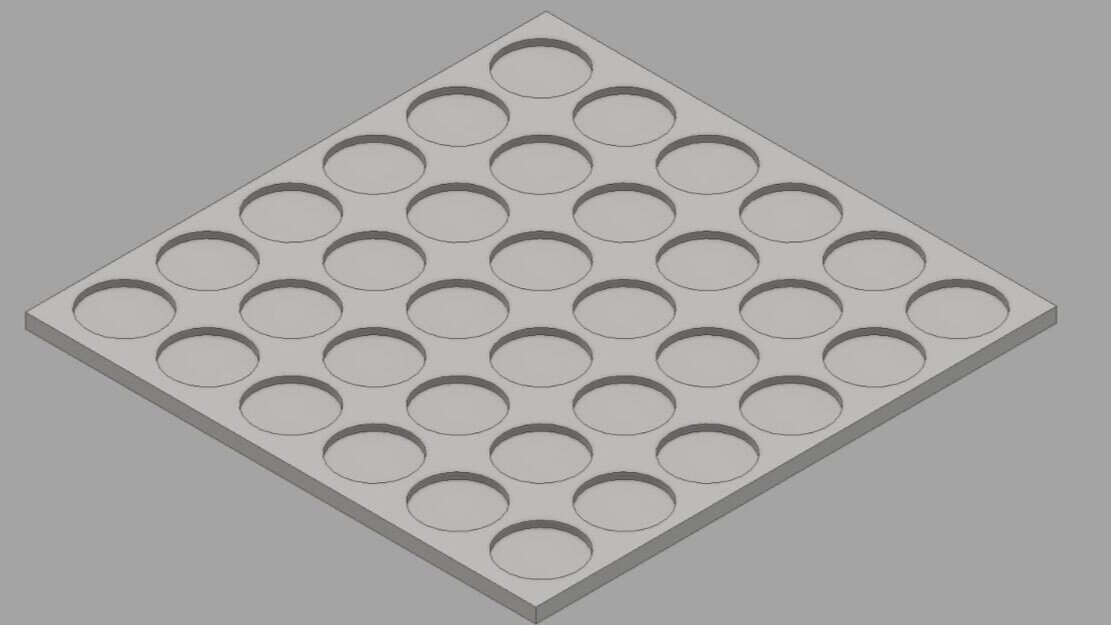
The DART (Drosophila ARousal Threshold) software suite provides comprehensive analytical solutions for high-throughput behavioural experimentation.
We are continuously upgrading the original version of our software published in 2015 to accommodate researchers needs and making sure of its user friendly interface. We also create and supply standardised hardware including platforms for video tracking. This includes classical 1D environments for sleep and circadian rhythm studies, and 2D environments for analysing open-field behaviour. In combination with these platforms, we supply external motors operated by our own custom-built USB controlled devices which can be utilised to carefully examine arousal threshold and stimuli responses. Finally, we manufacture enclosures that can provide optogenetic and IR illumination. BFK also provides tailored-made analytical solutions within the DART software suite, continuously expanding its potential for your research. We create new functions which provide users with specific solutions to quantify your own unique experimental questions.
How deeply does your mutant sleep? the DART suite
In this article, we describe the DART system for the 1st time. Since 2015, we have been continuously updating the software and are now providing the associated hardware.
The DART suite is extremely versatile. It has been used for the study of general anaesthesia; as an alternative to the negative geotaxis climbing assay; a more precise strategy to look into the effects of aging;
Software development:
We kept the DART software up to date with the newest Matlab release, upgrade tracking, and enhance the user-friendly interface to offer more flexibility
User-Friendly Graphical Interface
Automatic tracking and manual correction allows for greater accuracy and fidelity of results.
Intuitive user interface allows the user for greater control of experimental design and quantitative analysis.
Data Analysis
Although DART comes pre-packaged with several basic analysis functions, we can create customisable analysis functions (visualisation, statistics and data output) to suit your investigation needs.
New Hardware
We are offering a wide range of hardware specifically design for running behavioural experiment using DART.
Plug and Play Infra-red camera from 15 to 60fps
1D platform for sleep and circadian experiments
2D or Y-shaped platforms
Motor controller
White-light enclosure for circadian experiments
Optogenetic device with Red-Green-Blue opsin activation
DART Set-up
To run the DART system you need:
We manufacture 1D environments that can hold up to 20 Trikinetic tubes (with vibrational motors).
We also manufacture 2D environments of multiple dimension (with/without vibrational motors).
A computer for running DART and data storage. We can assist you in choosing the right machine for your needs.
A recording device. DART is designed to run with a simple webcam, but more sophisticated recording devices can be used (depending on the size/scale of your experiment).
The environment or apparatus for your model organism.
A Digital to Analog Converter (DAC) to provide highly controlled external stimuli (vibrational motors, chemical odours, lights, etc.)
enclosure
All in one enclosure including recording system (with/without optogenetic functionality) and controllers to connect to the computer and 1D/2D environments.
Hight-throughput setup
Possibility to add a compact USB3 camera (2592 x 1944 px, pixel size 2.2 µm, 48.0 fps)
Hight-throughput setup composed of 6 platforms with motors and IR-lights underneath for better image quality.
Each platform can be independently controlled and can hold 18 tubes.
Comes with a surrounding frame
optogenetic platform (rgb)
DART References
1. Busack I, Jordan F, Sapir P, Bringmann H. The OptoGenBox - a device for long-term optogenetics inC. elegans. Journal of Neurogenetics. 2020.
2. Smith BR, Macdonald SJ. Dissecting the Genetic Basis of Variation in Drosophila Sleep Using a Multiparental QTL Mapping Resource. Genes. 2020;11(3).
3. Pamboro ELS, Brown EB, Keene AC. Dietary fatty acids promote sleep through a taste-independent mechanism. Genes Brain and Behavior. 2020;19(4).
4. Ertekin D, Kirszenblat L, Faville R, van Swinderen B. Down-regulation of a cytokine secreted from peripheral fat bodies improves visual attention while reducing sleep in Drosophila. PLoS biology. 2020;18(8).
5. Brown EB, Shah KD, Faville R, Kottler B, Keene AC. Drosophila insulin-like peptide 2 mediates dietary regulation of sleep intensity. Plos Genetics. 2020;16(3).
6. Bridi JC, Ludlow ZN, Kottler B, Hartmann B, Vanden Broeck L, Dearlove J, et al. Ancestral regulatory mechanisms specify conserved midbrain circuitry in arthropods and vertebrates. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(32):19544-55.
7. Bi J, Wang YF. The effect of the endosymbiont Wolbachia on the behavior of insect hosts. Insect Science. 2020;27(5):846-58.
8. Tuxworth RI, Taylor MJ, Anduaga AM, Hussien-Ali A, Chatzimatthaiou S, Longland J, et al. Attenuating the DNA damage response to double-strand breaks restores function in models of CNS neurodegeneration. Brain Communications. 2019;1(1).
9. Troup M, Zalucki OH, Kottler BD, Karunanithi S, Anggono V, van Swinderen B. Syntaxin1A Neomorphic Mutations Promote Rapid Recovery from Isoflurane Anesthesia in Drosophila melanogaster. Anesthesiology. 2019;131(3):555-68.
10. Taylor MJ, Tuxworth RI. Continuous tracking of startled Drosophila as an alternative to the negative geotaxis climbing assay. Journal of Neurogenetics. 2019;33(3):190-8.
11. Scaplen KM, Mei NJ, Bounds HA, Song SL, Azanchi R, Kaun KR. Automated real-time quantification of group locomotor activity in Drosophila melanogaster. Scientific reports. 2019;9.
12. Rao SR, Olechnowicz SWZ, Kratschmer P, Jepson JEC, Edwards CM, Edwards JR. Small Animal Video Tracking for Activity and Path Analysis Using a Novel Open-Source Multi-Platform Application (AnimApp). Scientific reports. 2019;9.
13. Mazaud D, Kottler B, Goncalves-Pimentel C, Proelss S, Tuchler N, Deneubourg C, et al. Transcriptional Regulation of the Glutamate/GABA/Glutamine Cycle in Adult Glia Controls Motor Activity and Seizures in Drosophila. Journal of Neuroscience. 2019;39(27):5269-83.
14. Kottler B, Faville R, Bridi JC, Hirth F. Inverse Control of Turning Behavior by Dopamine D1 Receptor Signaling in Columnar and Ring Neurons of the Central Complex in Drosophila. Current Biology. 2019;29(4):567-+.
15. Kirszenblat L, Yaun R, van Swinderen B. Visual experience drives sleep need in Drosophila. Sleep. 2019;42(7).
16. Geissmann Q, Rodriguez LG, Beckwith EJ, Gilestro GF. Rethomics: An R framework to analyse high-throughput behavioural data. PloS one. 2019;14(1).
17. Geissmann Q, Beckwith EJ, Gilestro GF. Most sleep does not serve a vital function: Evidence from Drosophila melanogaster. Science Advances. 2019;5(2).
18. Dai XM, Zhow EX, Yang W, Zhang XH, Zhang WX, Rao Y. D-Serine made by serine racemase in Drosophila intestine plays a physiological role in sleep. Nature communications. 2019;10.
19. Coll-Tane M, Krebbers A, Castells-Nobau A, Zweier C, Schenck A. Intellectual disability and autism spectrum disorders 'on the fly': insights from Drosophila. Disease Models & Mechanisms. 2019;12(5).
20. Chen KF, Lowe S, Lamaze A, Kratschmer P, Jepson J. Neurocalcin regulates nighttime sleep and arousal in Drosophila. eLife. 2019;8.
21. Yurgel ME, Shah KD, Brown EB, Burns C, Bennick RA, DiAngelo JR, et al. Ade2 Functions in the Drosophila Fat Body To Promote Sleep. G3-Genes Genomes Genetics. 2018;8(11):3385-95.
22. Watanabe LP, Riddle NC. Measuring Exercise Levels in Drosophila melanogaster Using the Rotating Exercise Quantification System (REQS). Jove-Journal of Visualized Experiments. 2018(135).
23. Troup M, Yap MHW, Rohrscheib C, Grabowska MJ, Ertekin D, Randeniya R, et al. Acute control of the sleep switch in Drosophila reveals a role for gap junctions in regulating behavioral responsiveness. eLife. 2018;7.
24. Stahl BA, Peco E, Davla S, Murakami K, Moreno NAC, van Meyel DJ, et al. The Taurine Transporter Eaat2 Functions in Ensheathing Glia to Modulate Sleep and Metabolic Rate. Current Biology. 2018;28(22):3700-+.
25. Somers J, Luong HNB, Batterham P, Perry T. Deletion of the nicotinic acetylcholine receptor subunit gene D1 confers insecticide resistance, but at what cost? Fly. 2018;12(1):46-54.
26. Shaw RE, Kottler B, Ludlow ZN, Buhl E, Kim D, da Silva SM, et al. In vivo expansion of functionally integrated GABAergic interneurons by targeted increase in neural progenitors. Embo Journal. 2018;37(13).
27. Lamaze A, Kratschmer P, Chen KF, Lowe S, Jepson JEC. A Wake-Promoting Circadian Output Circuit in Drosophila. Current Biology. 2018;28(19):3098-+.
28. Kirszenblat L, Ertekin D, Goodsell J, Zhou YQ, Shaw PJ, van Swinderen B. Sleep regulates visual selective attention in Drosophila. Journal of Experimental Biology. 2018;221(24).
29. Karunanithi S, Troup M, van Swinderen B. Using Drosophila to Understand General Anesthesia: From Synapses to Behavior. In: Eckenhoff RG, Dmochowski IJ, editors. Chemical and Biochemical Approaches for the Study of Anesthetic Function, Pt A. Methods in Enzymology. 6022018. p. 153-76.
30. De Lazzari F, Bisaglia M, Zordan MA, Sandrelli F. Circadian Rhythm Abnormalities in Parkinson's Disease from Humans to Flies and Back. International Journal of Molecular Sciences. 2018;19(12).
31. Bi J, Sehgal A, Williams JA, Wang YF. Wolbachia affects sleep behavior in Drosophila melanogaster. Journal of Insect Physiology. 2018;107:81-8.
32. Aboudhiaf S, Alves G, Parrott S, Amri M, Simonnet MM, Grosjean Y, et al. LAT1-like transporters regulate dopaminergic transmission and sleep in Drosophila. Sleep. 2018;41(10).
33. Yap MHW, Grabowska MJ, Rohrscheib C, Jeans R, Troup M, Paulk AC, et al. Oscillatory brain activity in spontaneous and induced sleep stages in flies. Nature communications. 2017;8.
34. Velazquez-Ulloa NA. A Drosophila model for developmental nicotine exposure. PloS one. 2017;12(5).
35. Tougeron K, Abram PK. An Ecological Perspective on Sleep Disruption. American Naturalist. 2017;190(3):E55-E66.
36. Stahl BA, Slocumb ME, Chaitin H, DiAngelo JR, Keene AC. Sleep-Dependent Modulation of Metabolic Rate in Drosophila. Sleep. 2017;40(8).
37. Qiu S, Xiao CF, Robertson RM. Different age-dependent performance in Drosophila wild-type Canton-S and the white mutant w1118 flies. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2017;206:17-23.
38. Murphy KR, Park JH, Huber R, Ja WW. Simultaneous measurement of sleep and feeding in individual Drosophila. Nature Protocols. 2017;12(11):2355-66.
39. Machado DR, Afonso DJS, Kenny AR, Ozturk-Colak A, Moscato EH, Mainwaring B, et al. Identification of octopaminergic neurons that modulate sleep suppression by male sex drive. eLife. 2017;6.
40. Harbison ST, Negron YLS, Hansen NF, Lobell AS. Selection for long and short sleep duration in Drosophila melanogaster reveals the complex genetic network underlying natural variation in sleep. Plos Genetics. 2017;13(12).
41. Geissmann Q, Rodriguez LG, Beckwith EJ, French AS, Jamasb AR, Gilestro GF. Ethoscopes: An open platform for high-throughput ethomics. PLoS biology. 2017;15(10).
42. Ferguson L, Petty A, Rohrscheib C, Troup M, Kirszenblat L, Eyles DW, et al. Transient Dysregulation of Dopamine Signaling in a Developing Drosophila Arousal Circuit Permanently Impairs Behavioral Responsiveness in Adults. Frontiers in Psychiatry. 2017;8.
43. Dubowy C, Sehgal A. Circadian Rhythms and Sleep in Drosophila melanogaster. Genetics. 2017;205(4):1373-97.
44. Beckwith EJ, Geissmann Q, French AS, Gilestro GF. Regulation of sleep homeostasis by sexual arousal. eLife. 2017;6.
45. Allada R, Cirelli C, Sehgal A. Molecular Mechanisms of Sleep Homeostasis in Flies and Mammals. Cold Spring Harbor Perspectives in Biology. 2017;9(8).
46. Murphy KR, Deshpande SA, Yurgel ME, Quinn JP, Weissbach JL, Keene AC, et al. Postprandial sleep mechanics in Drosophila. eLife. 2016;5.
47. Garbe DS, Vigderman AS, Moscato E, Dove AE, Vecsey CG, Kayser MS, et al. Changes in Female Drosophila Sleep following Mating Are Mediated by SPSN-SAG Neurons. Journal of Biological Rhythms. 2016;31(6):551-67.
48. Borbely AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. Journal of sleep research. 2016;25(2):131-43.
49. Aulsebrook AE, Jones TM, Rattenborg NC, Roth TC, Lesku JA. Sleep Ecophysiology: Integrating Neuroscience and Ecology. Trends in Ecology & Evolution. 2016;31(8):590-9.
50. Zalucki OH, Menon H, Kottler B, Faville R, Day R, Bademosi AT, et al. Syntaxin1A-mediated Resistance and Hypersensitivity to Isoflurane in Drosophila melanogaster. Anesthesiology. 2015;122(5):1060-74.
51. Zalucki O, Day R, Kottler B, Karunanithi S, van Swinderen B. Behavioral and electrophysiological analysis of general anesthesia in 3 background strains of Drosophila melanogaster. Fly. 2015;9(1):7-15.
52. Garbe DS, Bollinger WL, Vigderman A, Masek P, Gertowski J, Sehgal A, et al. Context-specific comparison of sleep acquisition systems in Drosophila. Biology Open. 2015;4(11):1558-68.
53. Bai L, Sehgal A. Anaplastic Lymphoma Kinase Acts in the Drosophila Mushroom Body to Negatively Regulate Sleep. Plos Genetics. 2015;11(11).